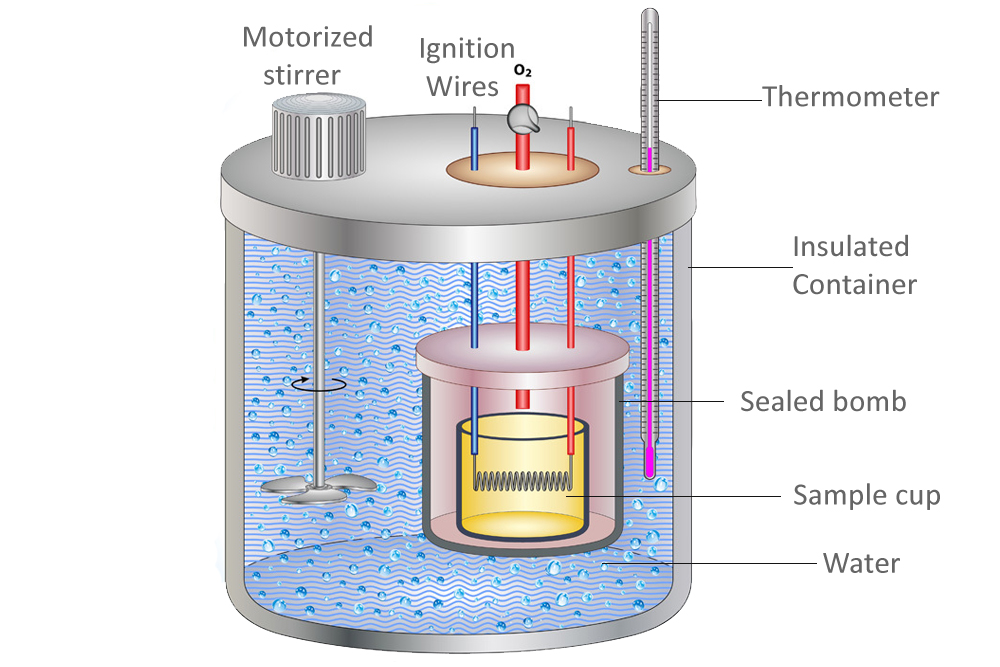
Proper Use Of Calorimeter . To do so, the heat is exchanged with a calibrated object. general chemistry students often use simple calorimeters constructed from polystyrene cups (figure 5.12). calorimetry is used to measure amounts of heat transferred to or from a substance. apply the first law of thermodynamics to calorimetry. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter.
from www.scienceabc.com
calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object. general chemistry students often use simple calorimeters constructed from polystyrene cups (figure 5.12). a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. apply the first law of thermodynamics to calorimetry. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for.
Molar Heat Capacity Definition, Formula, Equation, Calculation
Proper Use Of Calorimeter apply the first law of thermodynamics to calorimetry. To do so, the heat is exchanged with a calibrated object. calorimetry is used to measure amounts of heat transferred to or from a substance. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. apply the first law of thermodynamics to calorimetry. general chemistry students often use simple calorimeters constructed from polystyrene cups (figure 5.12). a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter.
From scienceinfo.com
Calorimeter Definition, Types and Uses Proper Use Of Calorimeter general chemistry students often use simple calorimeters constructed from polystyrene cups (figure 5.12). calorimetry is used to measure amounts of heat transferred to or from a substance. apply the first law of thermodynamics to calorimetry. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. Compare heat flow from hot. Proper Use Of Calorimeter.
From www.youtube.com
FOOD TESTS AND CALORIMETER EXPERIMENT YouTube Proper Use Of Calorimeter apply the first law of thermodynamics to calorimetry. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. To do so, the heat is exchanged with a calibrated object. calorimetry is used to measure amounts of heat. Proper Use Of Calorimeter.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID1875569 Proper Use Of Calorimeter To do so, the heat is exchanged with a calibrated object. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. general chemistry students often use simple calorimeters constructed from polystyrene cups (figure 5.12). calorimetry. Proper Use Of Calorimeter.
From www2.mrc-lmb.cam.ac.uk
Isothermal titration calorimetry to measure protein/peptide/lipid Proper Use Of Calorimeter Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. To do so, the heat is exchanged with a calibrated object. apply the first law of thermodynamics to calorimetry. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. calorimetry is used to measure amounts. Proper Use Of Calorimeter.
From www.tessshebaylo.com
Equation For Calorimetry Tessshebaylo Proper Use Of Calorimeter calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. apply the first law of thermodynamics to. Proper Use Of Calorimeter.
From www.collegesearch.in
Principle of Calorimetry Definition, Formula, Principle, Types Proper Use Of Calorimeter a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. To do so, the heat is exchanged with a calibrated object. calorimetry is used to measure amounts of heat transferred to or from. Proper Use Of Calorimeter.
From www.indiamart.com
Bomb Calorimeter with Automatic Calculation, For Coal And Briquette Proper Use Of Calorimeter calorimetry is used to measure amounts of heat transferred to or from a substance. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. To do so, the heat is exchanged with a calibrated object. apply the first law of thermodynamics to calorimetry. Compare heat flow from hot to cold objects. Proper Use Of Calorimeter.
From evulpo.com
evulpo Calorimetry Proper Use Of Calorimeter calorimetry is used to measure amounts of heat transferred to or from a substance. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. general chemistry students often use simple calorimeters constructed from polystyrene cups (figure 5.12). apply the first law of thermodynamics to calorimetry. To do. Proper Use Of Calorimeter.
From www.labtesting-equipment.com
ISO 5660 AC220V Cone Calorimeter For Building Materials Testing Proper Use Of Calorimeter calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. To do so, the heat is exchanged with a calibrated object. calorimetry is used to measure amounts of heat transferred to or from a substance. a calorimeter is a device used to measure the amount of heat involved in a chemical. Proper Use Of Calorimeter.
From www.science-revision.co.uk
Calorimetry Proper Use Of Calorimeter calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. To do so, the heat is exchanged with a calibrated object. calorimetry is used to measure amounts of heat transferred to or from a substance. a calorimeter is a device used to measure the amount of heat involved in a chemical. Proper Use Of Calorimeter.
From www.youtube.com
How to find calorimeter constant YouTube Proper Use Of Calorimeter apply the first law of thermodynamics to calorimetry. calorimetry is used to measure amounts of heat transferred to or from a substance. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.. Proper Use Of Calorimeter.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Proper Use Of Calorimeter Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. To do so, the heat is exchanged with a calibrated object. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. apply the first law of thermodynamics to calorimetry. general chemistry students often use simple. Proper Use Of Calorimeter.
From www.researchgate.net
Differential scanning calorimetry (DSC) curve of PVDF demonstrating the Proper Use Of Calorimeter calorimetry is used to measure amounts of heat transferred to or from a substance. apply the first law of thermodynamics to calorimetry. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. To do so, the heat is exchanged with a calibrated object. Compare heat flow from hot. Proper Use Of Calorimeter.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation Proper Use Of Calorimeter calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. general chemistry students often use simple calorimeters constructed from polystyrene cups (figure 5.12). apply the first law of thermodynamics to calorimetry. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. To do so, the. Proper Use Of Calorimeter.
From www.tessshebaylo.com
Equation For Constant Volume Calorimetry Tessshebaylo Proper Use Of Calorimeter To do so, the heat is exchanged with a calibrated object. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. calorimetry is used to measure amounts of heat transferred to or from a substance. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Web. Proper Use Of Calorimeter.
From bettyelevisxo.blob.core.windows.net
Fundamentals Of Calorimetry Lab Proper Use Of Calorimeter calorimetry is used to measure amounts of heat transferred to or from a substance. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. apply the first law of thermodynamics to calorimetry. To do so, the heat is exchanged with a calibrated object. calorimetry is the set of techniques used. Proper Use Of Calorimeter.
From www.studypool.com
SOLUTION Calorimetry lab gizmo all answers correct Studypool Proper Use Of Calorimeter general chemistry students often use simple calorimeters constructed from polystyrene cups (figure 5.12). Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. apply the first law of thermodynamics to calorimetry. To. Proper Use Of Calorimeter.
From chemlab.truman.edu
Parr 1341 Bomb Calorimeter Chem Lab Proper Use Of Calorimeter calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. general chemistry students often use simple calorimeters constructed from polystyrene cups (figure 5.12). To do so, the heat is exchanged with a calibrated object. calorimetry is. Proper Use Of Calorimeter.